|
 |
  |
| 홍합유래 바이오접착소재 개발 및 응용연구
|
1. 서론
많은 해양 부착 생물들 , 특히 해양 무척추동물들은 그들의 흡착기관을 이용해 수중 기저에 단단하게 부착함으로써 동적인 바다환경에서 살아간다 . 이러한 부착성 해양생명체 중에서 홍합과 따개비는 바다에서 쉽게 볼 수 있을 정도로 숫자가 많기 때문에 해양접착제 연구의 주요 대상이 되어 왔다 . 따개비 접착의 연구가 여전이 초기 단계에 머물고 있는데 반하여 [1,2], 홍합 접착에 대한 생화학적 연구는 약 25 년 간 축적되어 왔다 .
전 세계에 일반적으로 보급되고 있는 음식 재료인 홍합은 내수성 생체접착제의 잠재적 원천으로써 그 동안 많은 연구들이 이루어져 왔다 [3,4]. 홍합은 염도 , 습도 , 조류 , 난류 , 파도 등에 의해 특징지어지는 해양환경에서 그들 스스로가 수중 접착할 수 있게 기능적으로 분화된 접착제를 생산하여 분비한다 . 이들의 발에서부터 분비된 섬유다발로 구성된 족사 (thread) 를 사용하여 물속에서 물질표면에 강하게 접착한다 . 각각의 섬유 끝에는 내수성 접착제로 구성된 플라크 (plaque) 가 있어 젖은 고체 표면에 붙을 수 있다 (Figure 1). 이러한 홍합접착단백질은 이러한 흥미로운 현상들 때문에 잠재적인 내수성 생체접착제로 여겨져 왔다 [3,6-8]. 홍합접착단백질은 또한 가장 강력한 접착제 중 하나로 알려져 있다 . 홍합접착은 에폭시나 페놀수지와 같은 다른 고분자 기반의 접착제보다 훨씬 강력하면서도 휘어지며 유연하다 . 홍합접착단백질의 가장 흥미로운 점은 수중 환경에서 그 접착력을 유지한다는 것이다 . 모든 살아있는 유기체에서 접착은 수분의 존재 하에 일어나지만 화학접착제는 물에 의해 쉽게 분해된다 . 어떤 화학합성접착제도 수중환경에서 이와 비슷하게 적용되지 않는다 . 홍합접착단백질은 또한 플라스틱 , 유리 , 금속 , 테플론 및 생체물질 등의 많은 종류의 표면에 접착할 수 있다 . 홍합접착단백질 ( 추출되거나 유전자재조합 된 ) 의 다양한 고체 물질에의 접착에 대한 연구는 이미 많이 이루어져 왔다 [9-14]. 최근 연구에서 홍합접착단백질이 생체물질 , 특히 동물피부 또는 다양한 종류의 동물세포를 잘 부착할 수 있다는 것도 확인되었다 . 강력하고 물에 강한 홍합접착제는 이들이 세포나 조직접착제로써 이용될 수 있고 생분해성으로 환경친화적이라는 이점을 가지기 때문에 생물공학적으로 다양하게 응용될 수 있어 관심을 끌고 있다 [18]. 홍합접착단백질은 또한 인체에 무해하며 면역반응을 거의 유도하지 않기 때문에 의료용 접착제로 사용될 수 있다 [6,8,9,10]. 그러므로 홍합생체접착제를 상용화하기 위한 많은 노력들이 시도되었다.
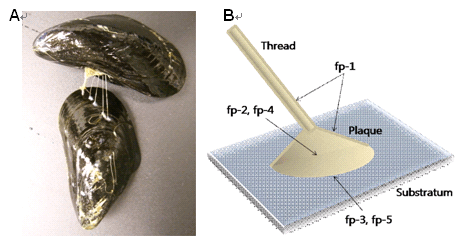 Figure 1. (A) Attachment of mussel to other mussel using byssal threads.
Figure 1. (A) Attachment of mussel to other mussel using byssal threads.
(B) Schematic illustration of a byssal thread and adhesive plaque. |
2. 홍합접착단백질
홍합접착단백질의 발견 및 물성 연구는 미국의 university of California, Santa Babara 의 J. Herbert Waite 교수 팀이 주도적으로 수행하고 있다 . 홍합의 족사와 접착 플라크는 몇몇의 다른 단백질들로 구성되어 있다 . 홍합접착단백질과 그 물성에 관한 연구는 족사 섬유로부터 3 가지의 콜라젠 (collagen) 단백질과 접착 플라크로부터 foot protein type 1 (fp-1) 에서 type 6 (fp-6) 의 6 가지 단백질이 규명되었다 (Table 1)[5,9,23-27]. 이 족사의 섬유는 perCols 로 알려진 콜라젠과 실의 융합된 형태로 주로 이루어져있다 . 이러한 족사 단백질을 발견하는데 주로 Mytilus edulis 라는 홍합종이 이용되었으나 Mytilus galloprovincialis , Mytilus coruscus , Mytilus californiaus 같은 홍합들에서도 이와 비슷한 홍합접착단백질의 유형과 아미노산 서열이 보고되었다 [10,18,28]. 흥미롭게도 이러한 족사 단백질은 폴리페놀 산화제 (polyphenol oxidase) 를 이용해 타이로신 (tyrosine) 기를 수화 (hydroxylation) 시켜 얻어지는 아미노산인 3,4-dihydroxyphenyl-L-alanine(Dopa)를 많이 포함한다 [5,23,24]. DOPA 의 곁가지에 있는 3,4-dihydroxyphenyl(catechol) 은 친수성 표면과 매우 강한 수소결합을 형성할 수 있으며 금속이온 , 금속 산화물 (Fe 3+ , Mn 3+ ), 반금속 ( 실리콘 ) 등과 강한 결합을 이룰 수 있다 [28-30]. 접착 경계면에 가까운 홍합접착단백질일수록 높은 비율의 DOPA 를 가지고 있다 [23-26]. Dopa 가 없는 홍합접착 단백질 유사물의 접착력이 크게 감소되는 것이 보고 되었다 [10,11,31]. 또한 Dopa 잔기는 Dopa-quinone 으로 산화되어 홍합접착단백질 분자들이 서로 가교되게 한다 [11,31].
Table 1. Mussel adhesive proteins
| Proteins |
Mass (kDa) |
DOPA (mol%) |
Features |
| fp-1 |
~110 |
~13 |
~80 times repeats of AKPSYPPTYK
two variants, basic proteinsurface coating
|
| fp-2 |
~40 |
~3 |
Cysteine rich
abundant in adhesion plaque |
| fp-3 |
~6 |
~20 |
arginine rich, hydroxyarginine
20-30 variants
surface adhesion |
| fp-4 |
~80 |
~5 |
histidine, lysine, arginine rich
Cu2+ binding |
| fp-5 |
~9.5 |
~30 |
phosphoserine
YK or YH repeats
surface adhesion |
| fp-6 |
~11 |
~4 |
cysteine rich (~11 mol%) |
Isolated from M. edulis and M. galloprovincialis except fp-6 that is discovered in M. californiaus
M. edulis (Mefp-1) 의 족사 단백질 fp-1 은 처음으로 발견된 홍합접착단백질로 현재까지 연구의 대부분의 주된 대상이 되어 왔다 [25,32]. Mefp-1 은 10 개의 아미노산 ( Ala-Lys-Pro-Ser-Tyr-Hyp-Hyp-Tyr-Dopa-Lys) 이 80 번 반복되는 특이한 구조를 가지고 있다 . 그래서 이것은 110 kDa 정도의 매우 큰 분자량을 가지고 있고 이의 Dopa 함유량은 10-15 mol% 이다 . 접착플라크의 전체 구조와 족사 섬유의 말단을 망라하고 있는 것은 대부분 Mefp-1 을 함유하고 있는 얇은 큐티클 (cuticle) 보호층이다 . 다른 Mytilus 홍합종들인 M. galloprovincialis [33], M. coruscus [34], M. californiaus [35] 들도 또한 10 개 아미노산이 반복되는 횟수와 아미노산 서열만 만 약간 다른 Mefp-1 유사단백질을 포함하고 있다 .
홍 합 접착 플라크의 대부분은 Mefp-2 와 Mefp-4 에 의해 형성된다 . (Figure 1B). Mefp-2 는 약 40 kDa 의 분자량과 ~3mol% 의 dopa 함유량을 가지고 있고 족사 플라크의 보전을 위한 중요한 특징인 다양한 단백질 분해에 대한 저항과 관련이 있다 . 또한 Mefp-2 는 족사에서 안정화의 역할을 할 것인 표피성장 (epidermal growth) 유사요소를 가지고 있다 [26]. Mefp-4 는 약 80 kDa 의 분자량과 ~4mol% 의 Dopa 함유량을 가지고 있고 매우 높은 정도의 histidine, lysine, arginine 을 가지고 있다 . 이 단백질은 족사 플라크 접합점에서 결합반응제로서의 역할을 하는 것으로 보인다 [36].
Mefp-3 와 Mefp-5 는 홍합의 접착 플라크와 접착 표면 사이의 경계면에 존재하고 (Figure 1B) 가장 높은 정도의 Dopa 함유량을 가지고 있다 . Mefp-3 는 ~20mol% 정도의 높은 Dopa 량을 가지고 있으며 많은 수의 arginine 잔기를 가지고 있는 basic 한 단백질이다 [23,36] 이는 ~6 kDa 로 가장 작은 질량의 단백질이지만 많은 변이체 (20~30) 을 가지고 있다 . 그러나 일반적으로 4~5 개의 변이체들이 플라크에서 발견되었다 . 특이적 변이체는 부착되는 표면종류에 따라 다를 것으로 예상들을 하였지만 실제로 표면 종류와 Mefp-3 의 발현 사이에는 아무 상관관계가 없는 것으로 보고되었다 [37]. M. galloprovincialis 는 Mgfp-3A 와 Mgfp-3B 의 변이체를 , M. californiaus 는 Mcfp-3-12 변이체를 포함하고 있다 [38]. Mefp-5 는 플라크 특이적 단백질이며 ~30mol% 의 dopa 량을 함유하고 있으며 이는 홍합접착단백질 중 가장 높은 값이다 [24]. 이 Mefp-5 는 또한 매우 많은 serine 잔기 (75 개 아미노산중 8 개 ) 를 가지고 있는데 serine 은 phosphoserine 으로 부분적으로 수정될 수 있다 . 이것은 8.3 pI 값 ( 인산화 ) 을 가지는 매우 많은 수의 lysine 잔기를 가지는 basic 한 단백질이며 ~9.5 kDa 의 질량을 가진다 . 그러나 홍합접착에서 phosphoserine 의 역할은 정확히 밝혀지지 않았다 [18]. 최근 M. galloprovincialis 로부터 Mgfp-5 의 cDNA 서열이 Mefp-5 와 ~94% 유사한 염기서열을 가지고 있음이 밝혀졌다 [10]. M. californianus 로부터 8.9 kDa 의 크기를 가진 Mcfp-5 도 발견되었다 [27].
새로운 유형의 홍합접착단백질 Mcfp-6 가 M. californianus 로부터 발견되었고 분자량은 11.6 kDa 였다 [27]. Mcfp-6 는 5mol% 미만의 작은 Dopa 양을 함유하고 있는 basic 한 단백질이다 . Mcfp-3 와 Mcfp-5 와 대조적으로 20mol% 로 tyrosine 이 우세하며 cystein 의 3 분의 1 이 thiolate 로 남아 11mol% 로 존재한다 [27]. 이로부터 Mcfp-6 가 dopa 가 많은 단백질과 대부분 플라크 단백질과의 표면 결합 사이에 점착 고리를 제공하는 것으로 믿어지고 있다 . |
3. 홍합접착단백질의 생산
홍합접착단백질이 많은 흥미로운 성질과 다양한 잠제적인 응용분야를 가지고 있음에도 불구하고 실용적인 응용은 생산이 매우 한정적이었기 때문에 제약이 되어 왔다 . 자연추출이 처음 상업적 목적으로 홍합접착단백질을 분리하기 위해 사용되었지만 이 과정은 매우 노동집약적이고 비효율적이어서 Mefp-1 1 그램을 얻기 위하여 10,000 개의 홍합이 요구된다 (Table 2)[39,40]. 현재 Mefp-1 와 Mefp-2 를 포함하는 추출 혼합물인 Cell-Tak™ (BD Bioscience Clontech) 과 추출된 Mefp-1 이 주로 포함된 MAP™ (Swedish BioScience Lab.) 이 상업적으로 이용 가능한 홍합접착단백질이다 . 낮은 추출 수득률과 높은 생산가가 용도를 제한하여 현재로는 세포나 조직 배양 접착제로만 사용되고 있다 . 그러므로 접착 시험과 실용적인 응용을 위한 많은 양의 접착단백질을 얻기 위하여 유전자 재조합 기술이 도입되었다 . fp-1 이 습한 환경에서 홍합의 접착에 주요한 단백질로 여겨졌기 때문에 주로 fp-1 의 유전적 생산이 대장균 , 효모 , 식물 등의 다양한 발현 시스템에서 시도되어 왔다 (Table 2)[9,39,41]. 그러나 접착능력을 가지며 실용적인 홍합접착단백질 ( 주로 완전한 크기의 fp-1) 을 생산하기 위한 시도는 높게 치우친 아미노산 조성 (5 개의 아미노산이 전체의 ~89% 차지 ; Table 3), 홍합과 타 발현 시스템간의 상이한 코돈 사용 (tRNA 이용 문제 ), 적은 발현양 등으로 인해 모두 실패하였다 [12,39,41]. fp-1 의 10 개 아미노산을 부분 (6~20) 으로 반복하고 합성 유전자 구조를 이용한 접착펩타이드들이 Saccharomyces cerevisiae [9] 와 대장균 [12,41,42] 에서 성공적으로 발현은 되었지만 그 접착 능력은 미미한 것으로 알려져 있다. 반복적인 식물세포벽 단백질유전자와 유사함으로 이용한 완전한 분자량을 가지는 Mgfp-1 의 담배에서의 발현도 시도되었으나 [43], 생산량과 접착능력 등은 그 후 보고되지 않았다 . 여기에다 낮은 생산성과 어려운 세포배양 기술과 같은 식물세포 배양의 본질적 문제가 산업용 생산을 위해 우선적으로 해결되어야 한다 . 홍합 초생세포의 배양 또한 시도되었으나 홍합접착단백질의 생산성은 보고되지 않았다 [44]. 그러므로 성공적인 대량생산 기술은 본 연구팀의 성공적인 연구 보고 전에는 개발되지 않았다고 결론을 내릴 수 있다 [10,13]. 1990 년대 중반부터 fp-1 의 합성폴리펩타이드 모사물 (mimics) 이 생산되었지만 이러한 화학기반의 모사물들은 자연 홍합접착단백질과 같은 생체적합성 (biocompatibility) 를 보이지는 않았다 [11,31,45,46].
Table 2. Production of mussel adhesive proteins and its mimics
| System |
Feature |
Ref |
| Natural extraction |
Mefp-1
Easy extraction process
Labor intensive (10,000 mussel/g)
High production cost
|
25 |
| Yeast |
Partial fp-1 (20 repeats)
Low production level
No hydroxylation
Low adhesion strength |
9 |
| E. coli |
Partial fp-1 repeats (6~20 repeats)
Inclusion body formation
High production is possible
No hydroxylation
Low adhesion strength |
12,41,42 |
Mgfp-5
Soluble expression
Low production level
Micro-scale adhesion tests
Strong adhesion strength |
10 |
Mgfp-3A
Low production level
Micro-scale adhesion tests
Medium adhesion strength
|
47 |
Hybrid fp-151
Inclusion body formation
High production level
Easy extraction process
Macro-scale adhesion tests
Strong adhesion strength |
13 |
| Plant |
Full fp-1
Low production level |
43 |
Polymer
based mimics |
Dopa-containing chemical-based polymer
Functional production
Biocompatibility problem |
11,31,45,46 |
Table 3.
Amino acid distribution of fp-1
| Amino acid
|
Number |
Mol% |
| Lys |
190 |
21.7 |
| Pro |
224 |
25.6 |
| Ser |
87 |
9.9 |
| Thr |
107 |
12.2 |
| Tyr |
168 |
19.2 |
| Others |
99 |
11.4 |
| Total |
875 |
100 |
최근 들어 Mefp-5 의 발견이 보고되었고 [24], 이 단백질이 현재까지 발견된 홍합접착단백질중에서 가장 높은 dopa 함량을 가지고 접착표면에 위치하기 떄문에 유력한 생체접착소재로 사용이 가능할 것으로 기대되었다 . 그러나 Mefp-5 의 접착 특성은 이 단백질의 획득량이 매우 제한적이기 때문에 조사가 이루어지지 못하고 있었다 . 이에 본 연구팀에서는 우수한 접착능력을 가지는 Mgfp-5 와 Mgfp-3A 가 재조합 대장균 시스템에서 성공적으로 생산될 수 있다는 것을 보였다 [10,47]. 특히 재조합 Mgfp-5 는 다양한 표면에 코팅하여 quartz crystal microbalance(QCM), atomic force microscopy (AFM) 등의 미세 분석기기로 분석하였을 때 Cell-Tak™ 과 재조합 Mgfp-3A 보다 높은 접착능력을 보였다 [10,17]. 그러나 재조합 Mgfp-5 의 수용성 발현으로 인한 발현 유도 후 세포성장 저해로 인해 메우 낮은 생산성을 보였다 (Table 4). Mgfp-5 의 분리정제율 또한 매우 낮았다 . 또한 재조합 Mgfp-5 는 분리정제 후 수용액에서 매우 높게 불용성이 되는 문제점을 수반하였다 . 이에 따라 상업적 이용을 위해 요구되는 높은 농도의 용액 샘플을 얻는 것은 불가능했다 . 재조합 Mgfp-3A 는 Cell-Tak™ 의 접착력과 매우 비슷하였으나 Mgfp-3A 의 발현도 세포성장 저해를 일으켜 매우 낮은 생산성을 보였다.
Table 4. Comparison of several mussel adhesive proteins.
| Material |
Expression Yield a (%) |
Purification Yield b (%) |
Production
Yieldc (mg/L) |
Solubility c
(g/L |
Cell Adhesion |
Cell Spreadingd |
Ref |
| Mgfp-1 |
N/A e |
N/A |
N/A |
N/A |
Excellent (Cell-Tak) |
Medium (Cell-Tak) |
|
| Mgfp-5 |
~13 |
~7 |
~2 |
~1 |
Good |
Poor |
10,17 |
| fp-151 |
~40 |
~53 |
~1000 |
~300 |
Excellent |
Poor |
13 |
| fp-151-RGD |
~40 |
~53 |
~1000 |
~300 |
Excellent |
Excellent |
16 |
aBased on total cellular proteins
bBased on initial mussel adhesive protein
cAfter purification
dDeformation of the plasma membrane and formation of cell?substrate attachments
eNot applicable
이러한 초기 홍합접착단백질들의 문제점들을 극복하기 위해 Mgfp-1 10 개 반복 아미노산을 6 번 반복된 구조를 Mgfp-5 의 N- 말단과 C- 말단에 유전자 수준에서 연결시킨 새로운 하이브리드 (hybrid) 홍합접착단백질 fp-151 을 설계 및 구축하여 대장균에서 성공적으로 발현하였다 [13]. 하이브리드 홍합생체접착제 fp-151 은 매우 큰 생산성과 아세트산을 이용한 쉬운 분리정제법을 가진다 (Figure 2 & Table 4). 파일럿 규모 (200 L) 의 생산에서 fp-151 의 경제적인 대량생산의 가능성을 보였다 (1 리터 유가식 배양 당 1 g 의 정제 단백질 ). 정제된 유전자 재조합 fp-151 은 표면 코팅 , QCM, AFM 을 포함한 미세 단위 접착력 분석에서 재조합 Mgfp-5 와 비교할 만 하였다 . 실험용 플라스틱 소비품들의 접착이 시도되어 타이로시네이즈 (tyrosinase) 를 이용해 수정된 fp-151 는 쉽게 이러한 품목들에 10 분 안에 접착하였지만 완전한 가교에는 약 12 시간이 소요되었다 . 이 fp-151 단백질은 또한 부착성 세포와 비부착성 세포를 포함한 다양한 세포들의 유형에서 효과적인 접착력 (Table 3) 과 좋은 생체적합성을 보였다 [13]. 또한 정제 후 불용성 문제도 fp-151 로 해결되어 충분한 농도의 (~300 g/L) 접착용액 샘플이 가능하여 졌다 (Table 4). 그러므로 재조합 하이브리드 fp-151 의 대단위 (bulk-scale) 접착 테스트도 가능해졌다 . 양성 대조군으로써 상업적으로 이용가능한 조직 접착제인 피브린 (fibrin) 글루를 사용하였을 때 fp-151 의 접착력이 피브린 글루 (~0.2 MPa) 보다 약 4 배 (~0.8 MPa) 가량 큼을 알 수 있었다 이는 정사각 (10mm x 10mm) 소가죽 표면 위에 10 mg 의 샘플이 사용되었을 때의 결과이다 (Figure 4).
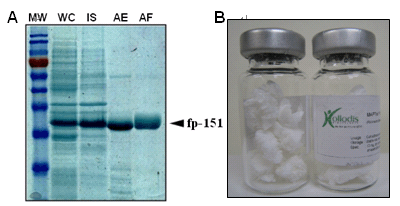 Figure 2. (A) Expression and purification of hybrid fp-151 in recombinant E. coli. Coomassie-blue-stained SDS-PAGE analysis was performed. Lanes: MW, protein molecular weight marker; WC, whole-cell sample; IS, insoluble cell debris fraction; AE, fraction extracted with 25% (v/v) acetic acid; AF, eluted fraction using His-tag affinity chromatography. (B) Purified and lyophilized recombinant hybrid fp-151 (Kollodis Bioscience).
Figure 2. (A) Expression and purification of hybrid fp-151 in recombinant E. coli. Coomassie-blue-stained SDS-PAGE analysis was performed. Lanes: MW, protein molecular weight marker; WC, whole-cell sample; IS, insoluble cell debris fraction; AE, fraction extracted with 25% (v/v) acetic acid; AF, eluted fraction using His-tag affinity chromatography. (B) Purified and lyophilized recombinant hybrid fp-151 (Kollodis Bioscience).
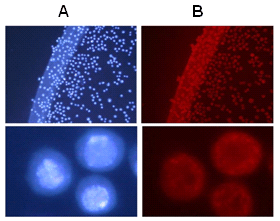
Figure 4. (A) Schematic of equipment used for the adhesion strength test, with cowhide adherends, in the Instron force measurement system with a 500 N (maximum capacity) load cell. (B) Comparison of the shear strength of hybrid fp-151 and fibrin glue in cowhide square pillars. The total amount of protein applied was 10 mg per cowhide square pillar (10 mm x 10 mm). The fixtures were cured at 45°C for 6 h in air. Each value and error bar represents the mean of four independent experiments and the standard deviation.
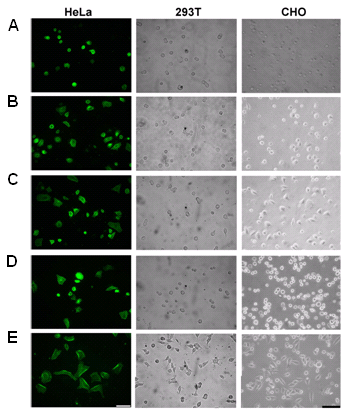
Figure 5. Cell spreading of human HeLa, human 293T, and Chinese hamster ovary (CHO) cells on (A) uncoated (NC), (B) PLL-, (C) Cell-Tak TM -, (D) fp-151-, and (E) fp-151-RGD-coated polystyrene surfaces. Bare polystyrene surfaces were coated with 7.5 μg of each sample and 5×10 4 cells (more than 95% of which were viable) in serum-free medium added to each coated well and incubated for 18 h. The scale bar is 100 m m.
본 연구팀을 또한 세포접착물질로서 특이적인 용도개발을 위하여 좀더 개선된 형태의 하이브리드 홍합접착단백질을 개발하였다 [16]. 양이온과 Dopa 를 통한 세포 접착 , 세포 표면의 integrin 을 통한 세포 접착을 모두 이용하기 위하여 fibronectin 의 세포 접착 위치에 있는 RGD 펩타이드를 fp-151 의 C- 말단에 연결시킨 fp-151-RGD 를 설계 , 구축하였다 . 이러한 새로운 하이브리드 fp-151-RGD 는 기존 fp-151 의 높은 생산성과 손쉬운 분리정제라는 이점을 가지면서도 다양한 인간 및 동물세포에 대해서 매우 뛰어난 세포접착능력과 세포 퍼짐 (spreading) 능력을 serum 이 없는 조건에서도 다른 상업적으로 이용되는 세포 접착 물질인 poly-L-lysine(PLL) ,Cell-Tak™ 보다 월등하게 보여주었다 (Figure 5). 그러므로 fp-151 과 fp-151-RGD 와 같은 새로운 하이브리드 홍합접착단백질들은 의학 , 생물 , 생물공학분야에서 성공적으로 사용할 수 있는 실용적인 생체접착제가 될 수 있는 가능성을 보여준다 . |
4. 맺음말
홍합접착 단백질은 수중환경에서 사용 가능하며 환경친화성을 가지고 있어 의료 분야에 사용이 가능하다 . 지난 25 년간 해양의 홍합으로부터 생체접착제를 개발하려는 시도들이 많이 이루어져 왔다 . 그러나 dopa 를 포함하는 홍합접착단백질의 실용적인 응용은 비경제적인 추출과 대규모 생산의 실패로 인해 심각하게 제한되어 왔다 . 이에 재조합 홍합접착단백질의 대량생산기술은 다양한 응용을 위한 실용적 생체접착제의 개발에 매우 중요하다 . 최근 개발된 하이브리드 홍합유래 바이오접착소재들은 이러한 우리의 꿈을 실현화 시켜줄 수 있을 것이다 . 그러므로 좀 더 실용적이고 좋은 물성을 가지는 바이오접착소재를 개발하기 위하여 다양한 접착단백질들을 함께 사용하여야 하며 이러한 개발된 바이오접착소재를 기반으로 하여 유전자 및 약물전달 , 방오 코팅제 , 수술용 실런트 등의 새로운 생물공학적 응용분야를 찾아 연구하여야 할 것이다.
|
5. 참고문헌
[1] Naldrett, M. J., Kaplan, D. L., Characterization of barnacle (Balanus eburneus and B. crenatus) adhesive proteins. Mar. Biol. 1997, 127, 629-635.
[2] Kamino, K., Novel barnacle underwater adhesive protein is a charged amino acid-rich protein constituted by a Cys-rich repetitive sequence. Biochem. J. 2001, 356, 503-507.
[3] Waite, J. H., Adhesion in byssally attached bivalves, Biol. Rev. 1983, 58, 209-231.
[4] Waite, J. H., Nature’s underwater adhesive specialist. Int. J. Adhesion Adhesives 1987, 7, 9-14.
[5] Rzepecki, L. M., Hansen, K. M., Waite, J. H., Characterization of a cystein-rich polyphenolic protein family from Mytilus edulis. Biol. bull. 1992, 183, 123-137.
[6] Dove, J., Sheridan, P., Adhesive protein from mussels: possibilites for dentistry, medicine, and industry. J. Am. Dent. Assoc. 1986, 112, 879.
[7] Pitman, M. I., Menche, D., Song, E. K., Ben-Yishay, A. et al., The use of adhesive in chondrocyte transplantation surgery: In vivo studies. Bull. Hospital Jt. Dis. Otrhop. Inst. 1989, 49, 213-220.
[8] Grande, D. A., Pitman, M. I., The use of adhesives in chondrocyte transplantation surgery: preliminary studies. Bull. Hospital Jt. Dis. Otrhop. Inst. 1988, 48, 140-148.
[9] Filpula, D. R., Lee, S. M., Link, R. P., Strausberg, S. L. et al., Structural and functional repetition in a marine mussel adhesive protein. Biotechnol. Prog. 1990, 6, 171-177.
[10] Hwang, D. S., Yoo, H. J., Jun, J. J., Moon, W.K. et al., Expression of functional recombinant mussel adhesive protein Mgfp-5 in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 3352-3359.
[11] Yu, M., Deming, T. J., Synthetic polypeptide mimics of marine adhesive. Macromolecules 1998, 31, 4739-4745.
[12] Kitamura, M., Kawakami, K., Nakamura, N., Tsumoto, K. et al., Expression of amodel peptide of a marine mussel adhesive protein in Escherichia coli and characterization of its structural and functional properties. J. Polym. Sci. 1999, 37, 729-736.
[13] Hwang, D. S., Gim, Y., Yoo, H. J., Cha, H.J., Practical recombinant hybrid mussel bioadhesive fp-151. Biomaterials 2007, 28, 3560-3568.
[14] Frank, B. P., Belfort, G., Atomic force microscopy for low-adhesion surfaces: thermodynamic criteria, critical surface, tension, and intermolecular forces. Langmuir 2001, 17, 1905-1912.
[15] Ninan, L., Monahan, J., Stroshine, R. L., Wilker, J. J. et al., Adhesive strength of marine mussel extracts on porcine skin. Biomaterials 2003, 24, 4091-4099.
[16] Hwang, D. S., Sim, S. B., Cha, H. J., Cell adhesion biomaterial based on mussel adhesive protein fused with RGD peptide. Biomaterials 2007, 28, 4039-4046.
[17] Hwang, D. S., Cha, H. J., Recombinant mussel adhesive protein Mgfp-5 as cell adhesion biomaterial. J. Biotechnol. 2007, 127, 727-735.
[18] Waite, J. H., Adhesion a la Moule. Integr. Comp. Biol. 2002, 42, 1172-1180.
[19] Saez, C., Pardo, J., Gutierrez, E., Brito, M. et al., Immunological studies of the polyphenolic proteins of mussels. Comp. Biochem. Physical. 1991, 98B, 569-572.
[20] Corne, K. J., Qin, X., Waite, J. H., Extensible collagen in mussel byssus: a natural block polymer. Science 1997, 277, 1830-1832.
[21] Qin, X., Corne, K. J., Waite, J. H., Tough tendons. Mussel byssus has collagen with silk-like domains. J. Biol. Chem. 1997, 272, 32623-32627.
[22] Waite, J. H., Qin, X., Corne, K. J., The peculiar collagens of mussel byssus. Matrix Biol. 1998, 17, 93-106.
[23] Papov, V. V., Diamond, T. V., Biemann, K., Waite, J. H., Hydroxyarginine-containing polyphenolic proteins in the adhesive plaques of the marine mussel Mytilus-edulis. J. Biol. Chem. 1995, 270, 20183-20192.
[24] Waite, J. H., Qin, X., Polyphosphoprotein from the adhesive pads of Mytilus edulis. Biochemistry 2001, 40, 2887-2893.
[25] Waite, J. H., Evidence for a repeating 3,4-dihydroxyphenylalanine- and hydroxyproline-containing decapeptide in the adhesive protein of the mussel, Mytilus edulis. J. Biol. Chem. 1983, 258, 2911-2915.
[26] Inoue, K., Takeuchi, Y., Miki, D., Odo, S., Mussel adhesive plaque proteins is a novel member of epidermal growth factor-like gene family. J. Biol. Chem. 1995, 270, 6698-6701.
[27] Zhao, H., Waite, J. H., Linking adhesive and structural proteins in the attachment plaque of Mytilus californianus. J. Biol. Chem. 2006, 281, 26150-26158.
[28] Deming, T. J., Mussel byssus and biomolecular materials. Curr. Opin. Chem. Biol. 1999, 3, 100-105.
[29] Sever, M. J., Weisser, J. T., Monahan, M., Srinvasan, S. et al., Metal-mediated cross-linking in the generation of a marine-mussel adhesive, Angew. Chem. Int. Ed. 2004, 43, 448-450.
[30] Monahan, J., Wilker, J. J., Specificity of metal ion crosslinking in marine mussel adhesives. Chem. Commun. 2003, 14, 1672-1673.
[31] Yu, M., Hwang, J., Deming, T. J., Role of L-3,4-dihydroxylphenylalanine in mussel adhesive proteins. J. Am. Chem. Soc. 1999, 121, 5825-5826.
[32] Waite, J. H., Tanzer, M. L., Polyphenolic substance of Mytilus edulis - novel adhesive containing L-DOPA and hydroxyproline. Science 1981, 212, 1038-1040.
[33] Inoue, K., Odo, S., The adhesive protein cDNA of Mytilus galloprovincialis encodes decapeptide repeats but no hexapeptide motif. Biol. Bull. 1994, 186, 349-355.
[34] Inoue, K., Takeuchi, Y., Takeyama, S., Yamaha, E. et al., Adhesive protein cDNA sequence of the mussel Mytilus coruscus and its evolutionary implications. J. Mol. Evol. 1996, 43, 348-356.
[35] Waite, J. H., Mussel glue from Mytilus californianus Conrad: a comparative study. J. Comp. Physiol. B 1986, 156, 491-496.
[36] Warner, S. C., Waite, J. H., Expression of multiple forms of an adhesive plaque protein in an individual mussel, Mytilus edulis. Mar. Biol. 1999, 134, 729-734.
[37] Floriolli, R. Y., von Langen, J., Waite, J. H., Marine surfaces and the expression of specific byssal adhesive protein variants in Mytilus. Mar. Biotechnol. 2000, 2, 352-363.
[38] Zhao, H., Robertson, N. B., Jewhurst, S. A., Waite, J. H., Probing the adhesive footprints of Mytilus californianus byssus. J. Biol. Chem. 2006, 281, 11090-11096.
[39] Morgan, D., Two firms race to derive profits from mussels’ glue. The Scientist 1990, 4, 1-6.
[40] Strausberg, R. L., Link, R. P., Protein-based medical adhesives. Trends Biotechnol. 1990, 8, 53-55.
[41] Salerno, A. J., Goldberg, I., Cloning, expression, and characterization of a synthetic analog to the biadhesive precursor protein of the sea mussel Mytilus edulis. Appl. Microbiol. Biotechnol. 1993, 39, 221-226.
[42] Wong, H. H., Kim, Y. C., Lee, S. Y., Chang, H. N., Effect of post-induction nutrient feeding strategies on the production of bioadhesive protein in Escherichia coli. Biotechnol. Bioeng. 1998, 60, 271-276.
[43] Marks, P., Mussel power. The New Scientist 1999, 164, 12.
[44] Takeuchi, Y., Inoue, K., Miki, D., Odo, S. et al., Cultured mussel foot cells expressing byssal protein genes. J. Exp. Zool. 1999, 283, 131-136.
[45] Dalsin, J. L., Hu, B., Lee, B. P., Messersmith, P. B., Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2005, 125, 4253-4258.
[46] Lee, H., Dellatore, S. M., Miller, W. M., Messersmith, P. B., Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426-430.
[47] Hwang, D. S., Gim, Y., Cha, H. J., Expression of functional recombinant mussel adhesive protein type 3A in Escherichia coli. Biotechnol. Prog. 2005, 21, 965-970. |
|
|
|
